“Off-the-shelf” TCR/CAR-T cell therapy
Why “off-the-shelf”?
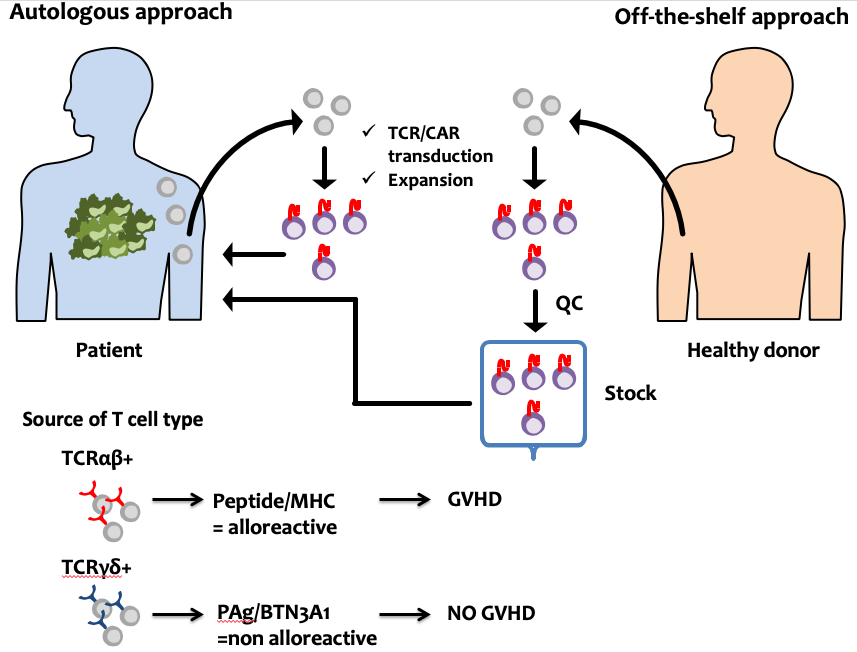
he successful TCR- and CAR-based adoptive T cell therapy thus far have used autologous T cells. However, such autologous approach has intrinsic problems including (i) logistical constraints in personalized manufacture, (ii) product variability and quality control, (iii) disease progression during manufacture, (v), and (iv) T cell dysfunction for broader application. “Off-the-shelf” TCR/CAR-T cells, defined as a biological agent that is pre-prepared in advance from healthy donors, validated and stored for ready to infuse as needed, may overcome these limitations.
Why Vγ9Vδ2 T cells
The major challenge in developing ‘off-the-shelf’ T cells is averting immune rejection in both host-versus-graft and graft-versus-host directions, but the latter is more important. Our approach to prevent GVHD is use of T cells with a defined TCR specificity lacking GVHD potential. T cells with TCR composed of Vγ9 and Vδ2 chains (Vγ9Vδ2 T cells) represent the major fraction in human peripheral blood and have the potent antitumor activity. Vγ9Vδ2 T cells respond to phosphoantigens (PAg) in a butyrophilin-3 A1 (BTN3A1), but not an MHC dependent manner. Therefore, Vγ9Vδ2 T cells appear less prone to alloreactivity that lowers the risk of GVHD. In addition, Vγ9Vδ2 T cells express activating Fc receptor (FcRIIIa/b, CD16) and exert antibody-dependent cellular cytotoxicity (ADCC), suggesting the possibility that infusing antibodies (Abs) that recognize additional TAAs can further activate CAR-Vγ9Vδ2 T cells and confer multiple targeting capacity to solid tumors with tumor antigen heterogeneity and loss. Taken together, Vγ9Vδ2 T cells appear to have potential as a source of an ‘off-the-shelf’ TCR/CAR-T cells (CAR-Vγ9Vδ2 T cells).
What we do
As a proof of concept, we use a second generation CD28 and CD3ζ endodomain-containing CAR and CEA as a target antigen with a favorable expression profile on many solid tumors. PBMC from healthy donors were stimulated using PTA and transduced with CAR using retroviral vector. Using an orthotopic mouse xenograft model, studies to prove the efficacy and safety of this approach are underway.



 TOP
TOP